根據電化學腐蝕原理,腐蝕過程中產生的電流大小可以代表腐蝕速率。由于陽極極化和陰極極化使腐蝕電池電位減小,從而降低腐蝕速率。產生陽極極化的主要原因是在腐蝕過程中,當溶液中有氧化劑時,在陽極表面產生了保護性的氧化膜,使金屬鈍化。其電位正移可達0.2~2V,可使腐蝕速率降低幾個數量級。
工(gong)業上廣(guang)泛應用的鐵、鉻(ge)、鎳、鈦及(ji)其合金(jin)的活化(hua)(hua)-鈍(dun)(dun)化(hua)(hua)曲(qu)線(xian)(xian)具有特殊的形式(shi),它(ta)們的活化(hua)(hua)-鈍(dun)(dun)化(hua)(hua)轉變(bian)的陽極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)如圖9.1所(suo)示。圖中有三個不同電(dian)(dian)(dian)(dian)(dian)化(hua)(hua)學行為區域:活化(hua)(hua)區A、鈍(dun)(dun)化(hua)(hua)區P和過(guo)鈍(dun)(dun)化(hua)(hua)區T。由于極(ji)(ji)化(hua)(hua)的作用,隨著腐蝕電(dian)(dian)(dian)(dian)(dian)流強(qiang)(qiang)度(du)的增加(jia),陽極(ji)(ji)電(dian)(dian)(dian)(dian)(dian)位(wei)E。升(sheng)高,當陽極(ji)(ji)極(ji)(ji)化(hua)(hua)曲(qu)線(xian)(xian)達(da)到(dao)最(zui)(zui)大(da)值,相應電(dian)(dian)(dian)(dian)(dian)極(ji)(ji)電(dian)(dian)(dian)(dian)(dian)位(wei)為Ep,電(dian)(dian)(dian)(dian)(dian)流強(qiang)(qiang)度(du)為Ip時,產生了陽極(ji)(ji)鈍(dun)(dun)化(hua)(hua),陽極(ji)(ji)過(guo)程受到(dao)極(ji)(ji)大(da)障礙,此時電(dian)(dian)(dian)(dian)(dian)流強(qiang)(qiang)度(du)突然下(xia)降到(dao)最(zui)(zui)小值I最(zui)(zui)小,Ep稱(cheng)為初始鈍(dun)(dun)化(hua)(hua)電(dian)(dian)(dian)(dian)(dian)位(wei),Ip稱(cheng)為臨(lin)界(jie)電(dian)(dian)(dian)(dian)(dian)流強(qiang)(qiang)度(du)。在很寬(kuan)的陽極(ji)(ji)電(dian)(dian)(dian)(dian)(dian)位(wei)范(fan)圍內(nei)極(ji)(ji)化(hua)(hua)時,一直(zhi)保持I最(zui)(zui)小的腐蝕電(dian)(dian)(dian)(dian)(dian)流強(qiang)(qiang)度(du),此時腐蝕速率大(da)大(da)降低(di),陽極(ji)(ji)處于鈍(dun)(dun)化(hua)(hua)區P。
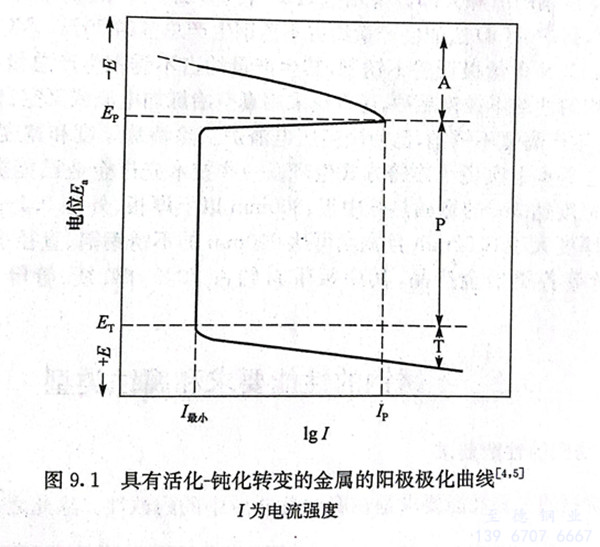
陽極電位超過(guo)(guo)Er后,腐(fu)蝕(shi)電流(liu)又增加,這種現象稱(cheng)為過(guo)(guo)鈍化(hua)。ET稱(cheng)為過(guo)(guo)鈍化(hua)電位,陽極處于過(guo)(guo)鈍化(hua)區(qu)T,此(ci)時金屬的腐(fu)蝕(shi)速率又增加。
根據具有(you)活化(hua)(hua)-鈍化(hua)(hua)轉變的(de)(de)金(jin)屬(shu)或合金(jin)的(de)(de)陽極極化(hua)(hua)曲線(xian)和陰極極化(hua)(hua)曲線(xian)的(de)(de)相對位置,可(ke)以分析(xi)該金(jin)屬(shu)和合金(jin)鈍化(hua)(hua)狀態的(de)(de)穩定性。



